
Pharmaceutical Validation Software Made Simple
Scalable, leading-edge validation technology that helps you drive efficiency, maintain data integrity, and stay in a state of audit readiness
Innovation Takes Time. Validation Shouldn't. Streamline GxP Compliance With Our Life Sciences Validation Software.
In the wake of increasing SaaS integration, today's IT and Quality teams are responsible for managing a surge of computer system validation (CSV) requirements, costing time and focus. Maintaining a state of data integrity and audit-readiness is crucial, but teams are increasingly asked to do more with less, creating operational bottlenecks and increasing risk. Tired of complex validation? Our GxP compliance solutions for life sciences delivers faster, simpler, and smarter results.
Taking the First Steps
Life sciences companies that are setting up a digital validation environment trust Sware to provide the guidance, expertise, critical thinking, and solutions-oriented mindset needed to build a foundation for lasting success.
Sware’s functional domain experts and out-of-the-box Res_Q platform help startups:
- Automate key workstreams and reduce manual tasks
- Maintain a state of audit-readiness, transparency, and high data integrity
- Easily scale to accommodate new workstreams

Taking the First Steps
Life sciences companies that are setting up a digital validation environment trust Sware to provide the guidance, expertise, critical thinking, and solutions-oriented mindset needed to build a foundation for lasting success.
Sware’s functional domain experts and out-of-the-box Res_Q platform help startups:
Automate key workstreams and reduce manual tasks
Maintain a state of audit-readiness, transparency, and high data integrity
Easily scale to accommodate new workstreams
Intelligent Validation for the
Future of Life Sciences
Life sciences companies trust Sware's pharmaceutical validation software to achieve remarkable results with less effort and fewer resources required. Sware helps life sciences IT and Quality leaders automate key workstreams, establish complete transparency and audit-readiness, and ensure data integrity at any scale. Our Res_Q platform unifies all validation processes and compliance modules (such as Veeva, DocuSign, and Box) to provide a single point of control, increasing efficiency and oversight from day one.

Explore Case Studies
Explore case studies from real clients. See how our customers were rescued from validation debt in their organizations.

"The time to execute validation workstreams went from days to hours with the implementation of Res_Q. We now have a proactive posture when it comes to cost and risk management. Res_Q is now a fundamental part of our bigger technology and compliance picture."
– Senior Director, Development Informatics
Read the Case Study
"We have shifted from CSV to CSA and now succeed with less documentation and more critical thinking. The difference pre- and post- Res_Q is simply night and day, we have accelerated our validation time by 75%!"
Read the Case Study
"Res_Q has saved us time and resources, reducing validation time by 30-40%. I wholeheartedly recommend Res_Q to fellow industry professionals seeking to enhance their validation processes."
– Sr. Director, IT Business Partner, GxP Systems
Read the Case Study![]()
“Validation workstreams that weren’t in Res–Q took days. Those same workstreams, once moved into the Res–Q system, take hours. It has helped us move from a reactive to proactive posture when it comes to cost and risk management and is a fundamental part of our bigger technology and compliance picture.”
Senior Director, Development Informatics
MOLECULAR MEDICINE COMPANY

Enabling Computer Software Assurance
When a company is ready to future-proof its validation activities in line with evolving regulatory expectations, Sware and Res_Q can facilitate the transition from computer system validation to computer system assurance (CSA), derisking validation and increasing efficiency by prioritizing tests based on impact and rejecting a “test everything” approach.
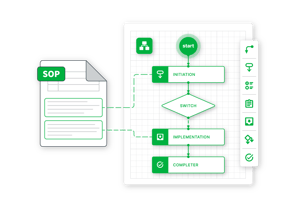
Could compliance risk be hiding in your organization's systems?
The intensifying pace of new software releases and regulatory expectations can make it hard to identify every liability. Take our short, 5-minute survey to gain a better understanding of your overall compliance risk.
“Validation workstreams that weren’t in Res_Q took days. Those same workstreams, once moved into the Res_Q system, take hours. It has helped us move from a reactive to proactive posture when it comes to cost and risk management and is a fundamental part of our bigger technology and compliance picture.”
Senior Director, Development Informatics
MOLECULAR MEDICINE COMPANY
“The old way involved more documentation and less critical thinking. The shift from CSV to computer systems assurance (CSA) turned this model on its head. Now, we succeed with less documentation and more critical thinking. With Res_Q, a project requiring 15 validation packages has now been reduced to less than 10, saving us significant time and allowing us to better direct our resources.”
Principal Consultant for IT, GxP Systems
PHARMACEUTICAL RESEARCH COMPANY
![]()
“The old way involved more documentation and less critical thinking. The shift from CSV to computer systems assurance (CSA) turned this model on its head. Now, we succeed with less documentation and more critical thinking. With Res–Q, a project requiring 15 validation packages has now been reduced to less than 10, saving us significant time and allowing us to better direct our resources.”
Principal Consultant for IT, GxP Systems
PHARMACEUTICAL RESEARCH COMPANY

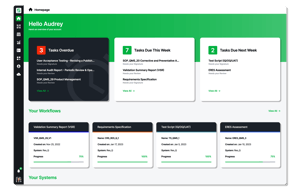
The Res_Q Platform
Sware’s Res_Q platform is a leading-edge solution that helps companies automate, unify, and accelerate validation through scalable, cloud-native, best-in-class technology. With Res_Q, life sciences and biotechnology companies can automate, unify, and accelerate their validation processes, reducing validation time by up to 70% and reducing lifetime CSV costs by up to 60%.
Trusted by:






