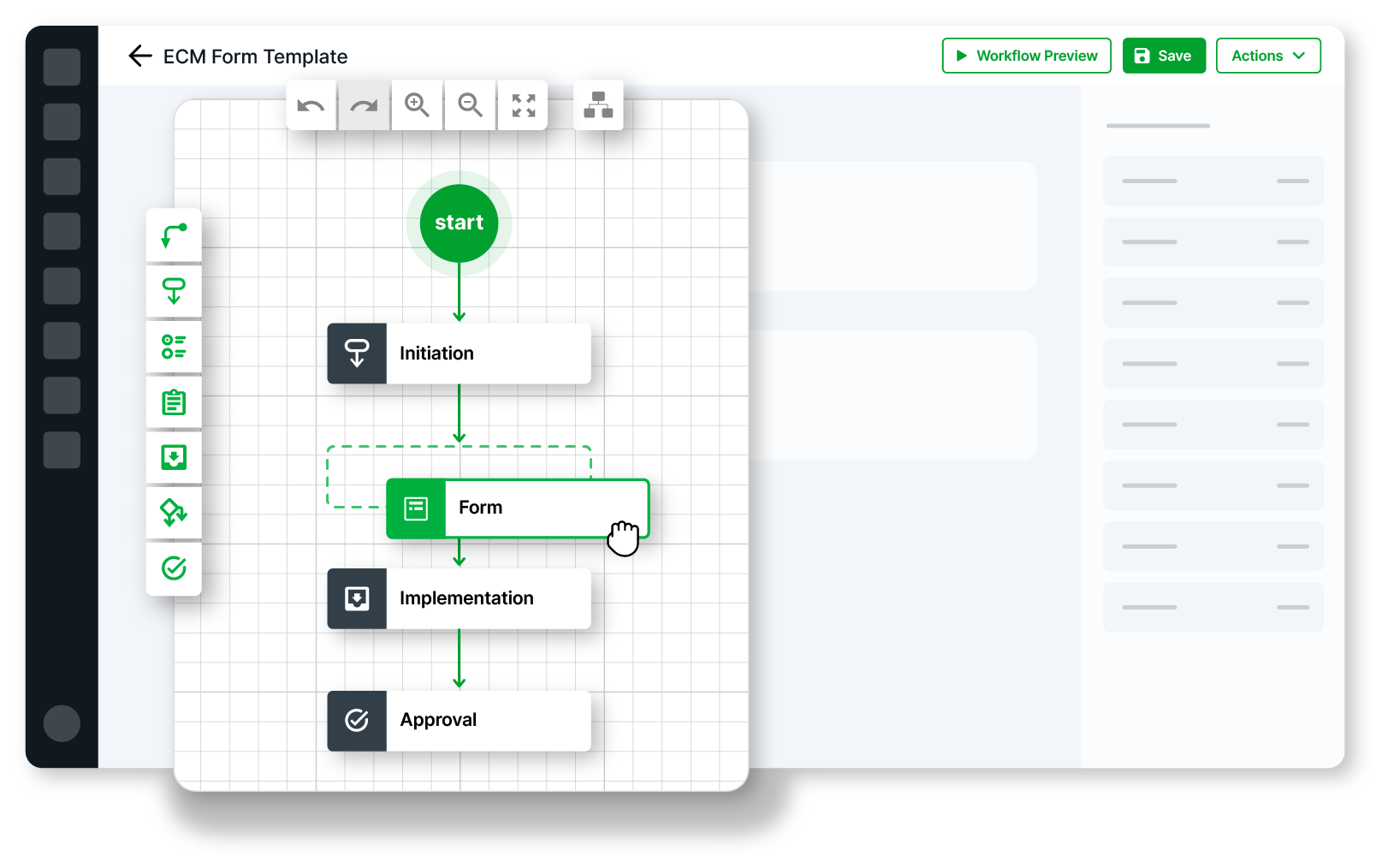
Building in GxP spaces should be easier
Navigating GxP presents challenges for developers that want to grow fast and go big in regulated sectors. Solve for success with GxPNext.

Simplify scoping, execution, and adoption
-
Scale rapidly with out-of-the-box, flexible technology
-
Seamlessly integrate a GxP validation solution into your SDLC toolkit
-
Optimize your validation strategy for customer use
-
Mitigate the impact of customer audits on your team

Achieve total audit-readiness and peace of mind
-
Automate traceability across requirements and test cases
-
Access audit-ready, customer-accessible document rooms
-
Generate best-in-class GxP validation evidence for customers
-
Work with the white-glove domain experts at Sware
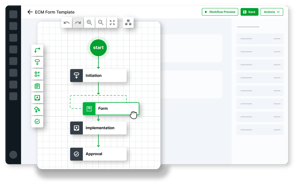
Accelerate time to revenue recognition
-
Automate software release validation workflows
-
Scalable, reusable content validation
-
Reduce your software release validation time by up to 40%
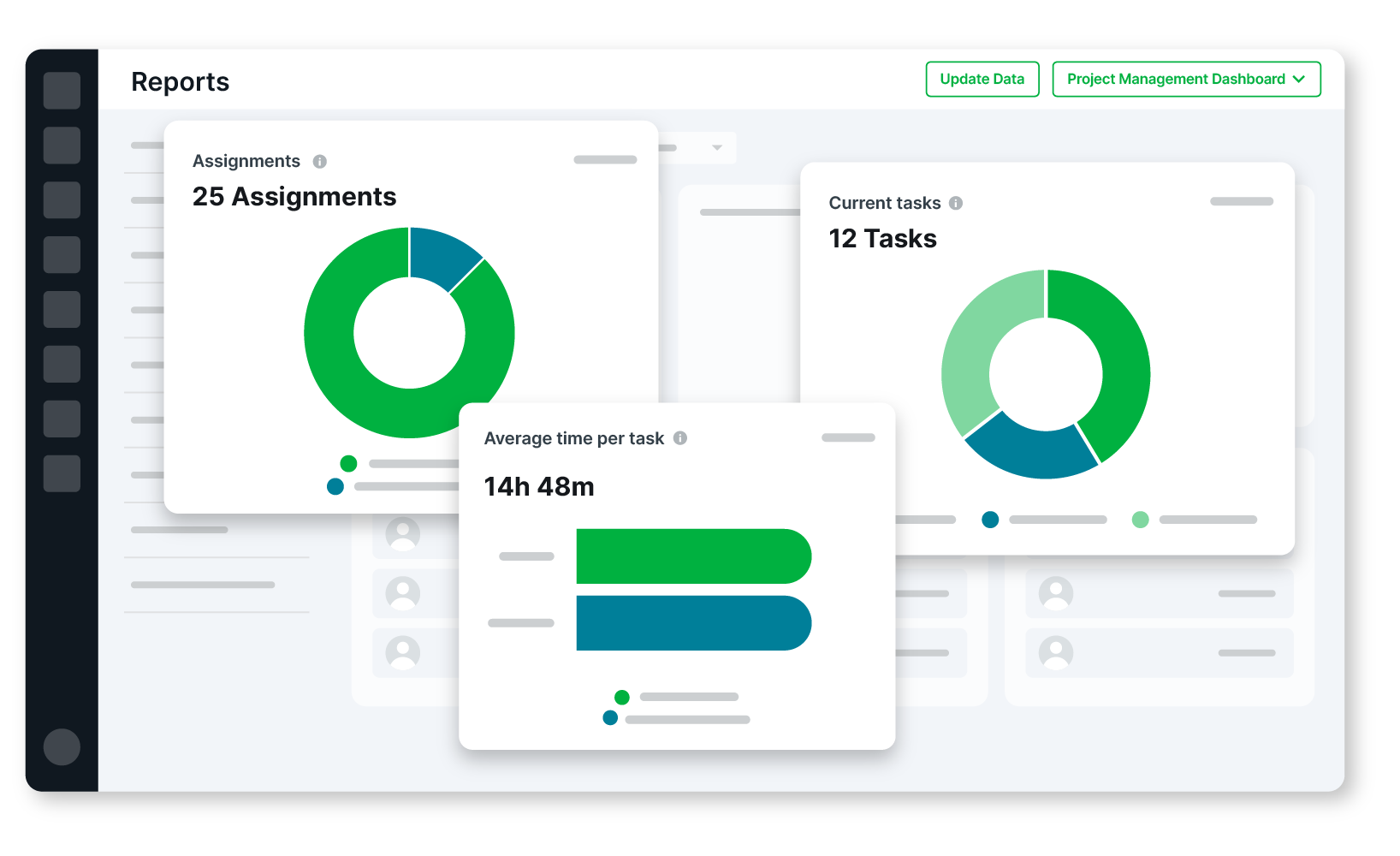
Execute customer projects with speed and quality
-
Automate customer validation workflows
-
Standardize and template your delivery process
-
Uncover data-driven insights with project management dashboards
-
Reduce your software implementation validation time by up to 50%


Learn how this SaaS provider transformed validation from a 7-engineer burden into a strategic differentiator for life sciences customers.
Our system is completely digitally mapped. As we built out that infrastructure, Sware provided invaluable support by bringing deep industry knowledge, experience, and connections to our project.”
Jason Siegrist
Chief Information Security Officer