
Res_Q: The Paperless Validation Software To Rescue You From Validation Debt
Meet the leading-edge validation solution that automates, unifies, and accelerates your success
Res_Q helps IT and quality leaders eliminate validation debt, achieve peace of mind and untangle valuable resources. Res_Q’s data-focused architecture and wide array of industry-supported applications prevent you from falling deeper into validation debt with every new software release.
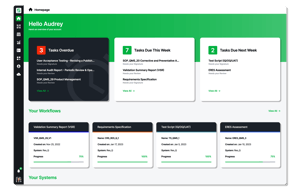
Res_Q reduces costs, prevents adoption delays, and streamlines cumbersome compliance requirements
Automate
release testing and GxP validation
Unify
validation processes in one app
Accelerate
validation time by up to 80%
Ensure
transparency and audit readiness
Less Validation Debt, More Peace of Mind
Features
Features
Res_Q Features

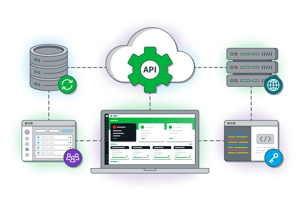
API-first Architecture
Open API approach unlocks bi-directional data flow that empowers system integrations, migration during onboarding, and more
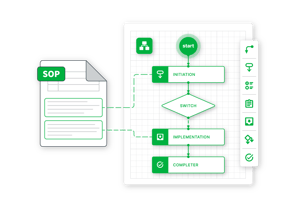
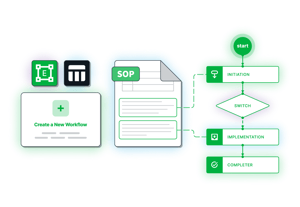
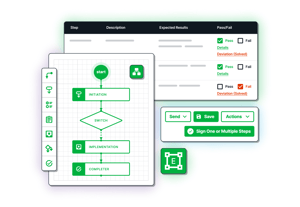
Workflow Engine
Configurable workflows to both match your operational requirements and integrate industry best practices
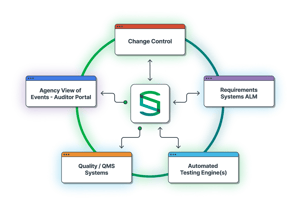
One Centralized App
All validation processes are controlled in a single system, serving all areas across the enterprise, including IT, manufacturing, lab systems, and more
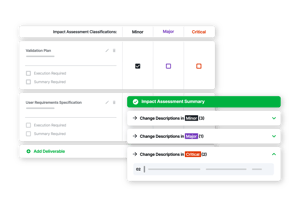
Intelligent Risk Assessments
Initiate workflows and assign workloads based on risk profile. Res_Q puts quality at the forefront without sacrificing speed
Full Lifecycle Management
Develop, execute, approve, store, and link to requirements within one centralized app

Cloud-powered and Secured
Hosted on AWS with separate database instances for each customer environment

30+ Compliance Modules
Over 30 integrated life sciences applications to accelerate implementation and ongoing release management

Controlled Auditor Access
Provide inspectors access directly to everything they need within Res_Q to accelerate and pass audits

One Centralized App
All validation processes are controlled in a single system, serving all areas across the enterprise, including IT, manufacturing, lab systems, and more
One Centralized App
All validation processes are controlled in a single system, serving all areas across the enterprise, including IT, manufacturing, lab systems, and more

Workflow Automation
Configurable workflows to both match your operational requirements and integrate industry best practices
Intelligent Risk Assessments
Initiate workflows and assign workloads based on risk profile. Res_Q puts quality at the forefront without sacrificing speed

Full Lifecycle Test Management
Develop, execute, approve, store, and link to requirements within one centralized app
Cloud-powered and Secured
Hosted on AWS with separate database instances for each customer environment
API-first Architecture
Open API approach unlocks bi-directional data flow that empowers system integrations, migration during onboarding, and more
Pre-built Compliance Modules
30+ connected life sciences applications to accelerate implementation and ongoing release management

Controlled Auditor Access
Provide inspectors access directly to everything they need within Res_Q to accelerate and pass audits
Featured Resources
The convergence of AI and Quality Management marks a paradigm shift in how Life Sciences organizations meet their GxP obligations. Examine key challenges—from compliance complexities and validation demands to talent shortages and risk management concerns.
Read the WhitepaperAs the life sciences landscape rapidly evolves, technology adoption demands agile validation methods to maintain GxP compliance. Learn how organizations can shift from project-based validation to a process-driven approach, integrating validation into their Quality Management System.
Read the WhitepaperManual computer software validation processes cost life sciences organizations up to 30% in additional project budget. As companies struggle to keep up with a surge of app and software integrations, they incur validation debt: the mounting cost of stretched resources, blanket testing, and missed GxP requirements.
Download the EbookAutomate. Unify. Accelerate.
Crush validation debt and reclaim valuable resources. Reach out to Sware to get started.