GxP Validation: From Business Bottleneck to Efficiency Engine
The purpose-built validation platform that simplifies and accelerates GxP processes across all your CDMO operations
Today's CDMOs face unprecedented pressures from all sides. As contract manufacturers in a global market, they must balance innovation with compliance while serving multiple client stakeholders. This creates unique challenges for both Quality and IT teams:
Quality Leadership Challenges:
-
Multiple client requirements leading to validation bottlenecks
-
Growing audit scrutiny demanding perfect record-keeping
-
Increasing regulatory complexity
-
Risk of compliance gaps affecting client relationships
IT Leadership Challenges:
-
Legacy systems struggling to scale with business growth
-
Resource drain from manual processes
-
Integration challenges across platforms
-
Technical debt accumulation
Transform Your CDMO Validation Process
Res_Q by Sware brings GxP validation platform technology into the modern era. Our data-centric, SaaS-based validation management system consolidates all your validation processes into a single, powerful platform that scales with your business.
Built by life sciences compliance experts who understand CDMO validation software requirements, Res_Q eliminates key burdens and bottlenecks through:
Automation
Visibility Across Operations
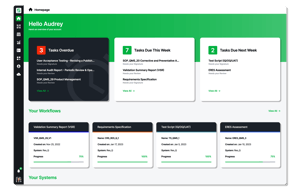
Go from Zero to Validated, Fast
Technology providers new to the GxP space trust GxPNext to enable critical features for a wide range of GxP use cases. GxPNext handles every step of the GxP validation process for every update and release, allowing customers a full range of change controls as they release validation packages.
Optimize Validation for Growth
Many providers have several pieces of the validation puzzle in place but need to optimize to accommodate customer growth. GxPNext helps get you there, enabling automated testing in SQA, robust, audited QMS, standardized validation product management, and a full range of managed services.
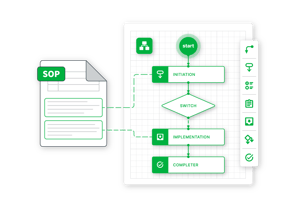
Transform and Evolve
Do you have a fully built product ecosystem with GxP validation capabilities, but need to evolve to meet your industry’s growing demands? GxPNext provides unparalleled scalability, flexibility, agility, and automation. Video-based testing evidence, electronic PQ test execution, risk tracking within product requirements, and other advanced features are available right out of the box.
"Sware provided invaluable support by bringing deep industry knowledge, experience, and connections to our project. Now, when I’m sitting across the table from a large life sciences client, I can speak their language and discuss compliance knowing that as audit processes evolve, we’ll be in a strong position to evolve with them."


Case Study: Enabling a CDMO to modernize validation
A growing CDMO faced validation challenges with paper-based legacy systems across multiple sites. Five releases behind in validation and lacking transparency, they needed a modern platform to maintain their position as an industry innovation leader.
Res_Q consolidated all validation documentation into a unified system, creating standardized processes across operations. The result: projected annual savings of 1.5 FTE in ERP validation efforts alone, modernized FDA audit readiness, and a foundation for continued process refinement and feature adoption.
"What impressed me most was their ability to provide both a sleek, modern validation tool and skilled resources who can hit the ground running to deliver results quickly. Their team really understands the unique challenges CDMOs face when it comes to GxP validation.”
– Vice President, Global IT
The Res_Q Difference
Res_Q delivers measurable value by reducing costs, preventing adoption delays, and streamlining cumbersome compliance requirements. Our comprehensive platform provides:
- Unified GxP validation platform manages validation across your entire operation
- Comprehensive compliance automation software for ERP, LIMS, and enterprise systems
- Pharmaceutical equipment qualification tracking and management
- Real-time validation status monitoring
- Risk-based validation approaches
- Streamlined analytical method validation
- Automated product validation workflows
- Equipment qualification processes
- CQV automation and tracking
- Built-in templates and risk assessment tools
- Centralized digital documentation
- Automated audit trails
- Version control and change management
- Electronic signatures and approvals
- Rapid audit response capabilities
- Access to CDMO validation specialists
- CDMO-specific implementation expertise
- Best practices guidance
- Ongoing process optimization
- Training and enablement

Case Study: Building validation infrastructure for a global CDMO
A leading life sciences manufacturer struggled with disconnected validation processes across 9 global facilities. With a new Microsoft Dynamics ERP implementation pending and inefficient legacy systems draining resources, they needed a modern validation solution to support growth.
Implementing Res_Q created a standardized validation platform across all facilities. The organization successfully validated 3 critical SaaS systems, gaining early FDA wins. Senior quality team members were freed for strategic initiatives, while establishing the company as tech-forward without compromising the bottom line.
No matter where you are in your validation journey, GxPNext enables innovation and growth
- Emerging
- Evolving
- Established
Go from Zero to Validated, Fast
Do you have a new GxP product and need a better validation solution to get to market?
- Replace paper-based validation processes with a fully paperless, digital solution.
- Increase efficiency, consistency, visibility and transparency across sites and users.
-

Optimize Validation for Growth
Do you have a foundation in place, but need to close the gaps to ensure you can grow?
GxPNext provides the templates, tools and best practices to take you to the next level of quality and efficiency.
-

Efficient Transformation
Do you have a fully built, GxP validated product ecosystem, but need to drive efficiency and address growing customer demand?
GxPNext provides unparalleled scalability, flexibility, and automation right out of the box. Cut the waste out of your process to speed timelines, reduce costs, and increase your level of Quality.
-

Learn More About the Res_Q Platform